Partnership develops wearable for COVID-19 early warning
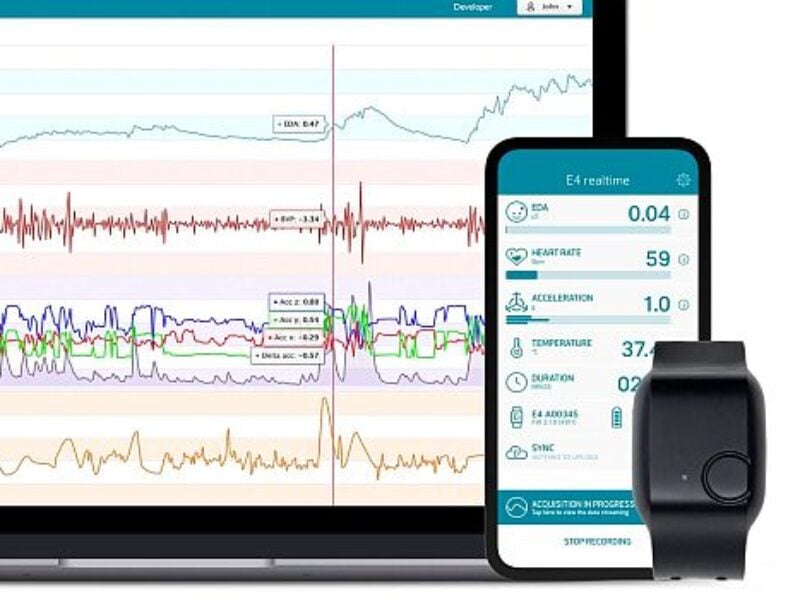
The system, named Aura, is completely noninvasive and uses the company’s wearable medical smartwatches, software, and artificial intelligence (AI) capabilities. Aura, says the company, will enable continuous and real-time insight into the likelihood of SARS-CoV-2 infection before symptoms present, and send a warning to the user and their healthcare provider.
Early last year the company and BARDA’s Division of Research, Innovation and Ventures (DRIVe) began to develop a digital biomarker that predicts respiratory infections. Preliminary findings, says the company, have been promising, showing a strong correlation between viral shedding and changes in a person’s physiology. Now it will be sponsored to run a validation trial specific to early detection of COVID-19.
The aim of the validation trial is to validate the company’s algorithm in real-life settings, with the participation of healthcare workers who are exposed to a high viral load while treating hospitalized COVID-19 patients. They will wear the company’s E4 medical-grade research wearable wristband for 30 days, during which their physiological data will be reviewed against daily nasopharyngeal (NP) samples and a daily qRT-PCR swab, ensuring the highest ground truth.
“This product introduces a new paradigm by empowering individuals and institutions with smart health monitoring, so that they will know early when they need to self-isolate and take care of themselves,” says Empatica CEO Matteo Lai. “Without BARDA’s leadership and foresight over the past year, our early detection algorithm would not have reached this pivotal stage of clinical validation, which will accelerate our request for FDA approval of Aura as a medical product for use by people at risk of contracting COVID-19.”
BARDA Acting Director, Gary Disbrow, Ph.D.,adds, “We anticipate that access to real-time and actionable health information will empower people to seek medical advice and care sooner, or to adopt behavioral changes such as temporary self-isolation that can help reduce the spread of COVID-19 and similar infections.”
Such early detection of disease, says the company, can protect frontline workers, reduce spread, and improve the overall public health response as lockdowns ease globally. CDC estimates suggest that 35% of infections are asymptomatic, making contact tracing and containment of the virus a challenge.
Meanwhile, the most infectious period could be one to three days before symptoms start, so even those patients who eventually display symptoms can still infect other people they interact with, before realizing they are ill. Digital biomarkers like Aura, says the company, can help efficiently triage patients, enabling more effective care and prioritization of cases, and potentially saving lives.
 If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News
If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News